Dear Patient,
You have recently been implanted with a joint prosthesis manufactured by Symbios Orthopédie S.A. In case of emergency or medical question, please contact your physician.

The Summary of Safety and Clinical Performance (SSCP) is available upon request at info@symbios.ch and will be made available in the European database on medical devices (Eudamed) when the functionality is available at https://ec.europa.eu/tools/eudamed for complete information about:

Patient identification (e.g., patient name or patient ID)

Date of implantation

Manufacturer of the device
Patient information website where a patient can obtain additional information on the medical product
Name and address of the implanting healthcare center or doctor where medical information about the patient may be found
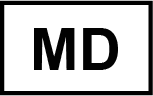
Device name

Reference number
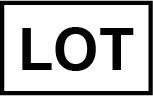
Lot number

Unique Device Identifier

Configuration number

Serial number

Case ID associated with an individual patient and surgery